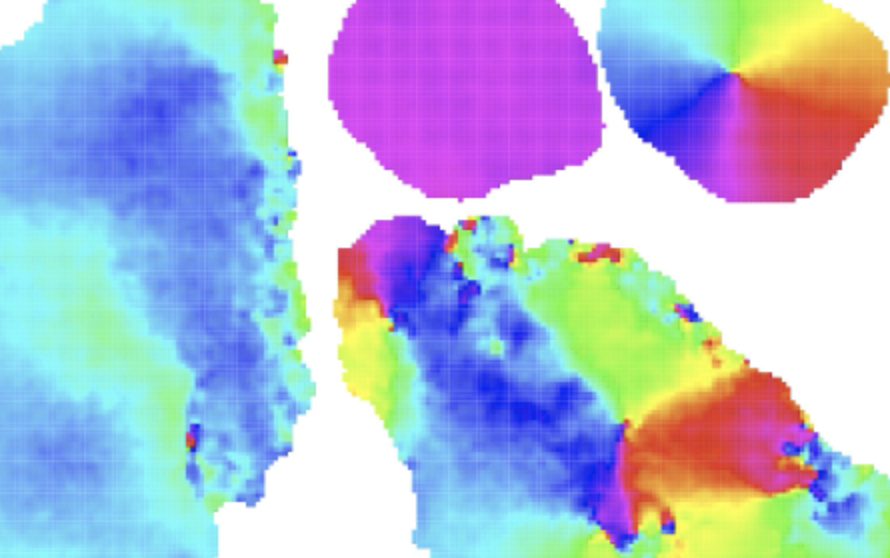
Early multicellular organisms without a nervous system behave like a collective
A new publication released by PNAS and authored by SCIoI PI Pawel Romanczuk (among others) explores how earliest multicellular organisms coordinate collective behavior – and SCIoI’s trip to Mexico last month has something to do with that.
Coordination between individuals is a fundamental challenge in collectives, and this is just as true for a country’s government as it is for simple multicellular organisms. How does a group of many cells coordinate itself into a body that performs coherent behavior? In more evolved animal groups, that’s the nervous system’s job. But if we go back to the beginning of the evolutionary tree and take a look at the first multicellular animals, we’ll notice that some of them are capable of moving in a coordinated manner even though they don’t have nervous systems. One example is the placozoan Trichoplax adhaerens, the simplest multicellular animal organism ever described. T. adherens is a type of marine amoeba with a body so undifferentiated that it not only lacks a nervous system, but also a mouth, stomach, muscles, blood, or veins. Yet, it is motile thanks to the collective action of thousands of cilia, small, hairlike structures that can beat in any direction, allowing motion.
This has brought researchers to wonder: how can a collection of cells move in a seemingly coordinated way if it doesn’t have a “brain”? In a new paper published in PNAS, SCIoI PI Pawel Romanczuk, together with Mircea Davidescu, Thomas Gregor, and Iain Couzin, investigated coordination among cells in T. adhaerens by measuring how well the collective moves together in different individuals of varying sizes. They found that the cells that compose the collective will initiate motion when the cells find themselves right at the border between a state of order and a state of chaos – that is, when they are in the so-called “state of criticality.” This phenomenon, which is known to occur in the animal brain, has also been observed in a Mexican fish collective described in another SCIoI paper. In that study, the researchers had observed the fish school perform fascinated, wave-like motions to escape predators whenever the group reached the stage of criticality, and the researchers had described that as a sort of “collective brain.”
The study combined detailed observation of T. adherens with a mathematical model that simulated its movement in the computer. Tracking under the microscope allowed to quantify how different parts of individual animals move with respect to each other when they explore their environment. Mathematically speaking, the simple multicellular animals were modeled as a self-organized collective of motile cells, i.e. cells able to move, coupled to each other. In computer simulations, researchers were able to generate predictions for the observed behaviors, and found that the behavior of the animals can be only explained if they operate at criticality.
If criticality is what allows Trichoplax adherens to coordinate the motion of its different parts, it also comes at a cost, and therefore the larger the animal (seen as a collective of cells,) the less organized its locomotion will appear. The scientists also went a step further and quantified the trade-off between increased size and motion coordination in T. adherens, and argued that there is a maximum size (1-2 mm) beyond which it is no longer possible for the system to move in a coordinated manner. This could be shedding new light on how and why larger organisms evolved hierarchical structures such as nervous systems.